News & Alerts


Systane Eye Drops Recall Owing to Possible Fungal Infection in the US
Zulkifal
December 26, 2024
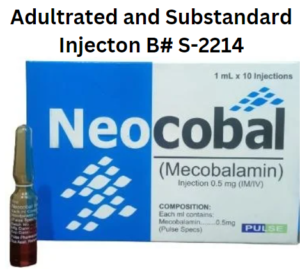
Recall Alert: Injection Neocobal (Mecobalamin) B# S-2214 By M/s Pulse Pharma Lahore, Pakistan
Zulkifal
December 26, 2024

Urgent Spurious & Falsified Drug Alert: Suspension Carefen 90 ml (Ibuprofen) B# CN-035
Zulkifal
December 25, 2024
